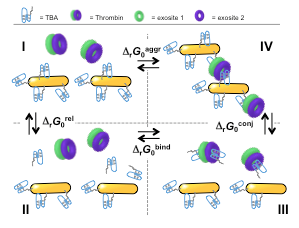
One challenge when using nanoparticles in biological applications is controlling interface between gold nanoparticles and biomolecules. Therefore, I decided to investigate the interface between nanoparticles and biomolecules, using thrombin-TBA binding as an illustration to develop a completely general thermodynamic model for quantification of the surface work on the nanoparticles due to ligand-receptor binding, which was published in the journal Small in 2011.
A simple thermodynamic framework can be used to quantify the interactions of proteins with biomolecules attached on the surface of nanoparticles.
Proteins can also non-specifically adsorb to the nanoparticles to form a “protein corona”, which can obscure the surface of the particles, and lead to unpredictable behavior. However, protein coronas around nanoparticles can be engineered to act as “sponges” for enhanced payload loading and release, representing an advance in nanobiotechnology for drug delivery applications, this work was published in ACS Nano, 2013.
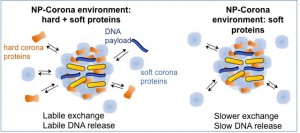
Protein coronas can bind to nanoparticles and they can be used for passive release of biomolecules that are nonspecifically absorbed to the protein corona